Synergistic silver/scandium catalytic spiroketalization of β-alkynyl ketones for the atom economical synthesis of skeletally diverse spirocyclic isochromenes†
Abstract
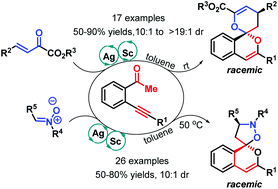
Two types of new synergistic silver/scandium catalytic spiroketalizations of β-alkynyl ketones have been achieved, delivering a variety of skeletally diverse spirocyclic isochromenes in generally good yields and good diastereoselectivity in an atom economical manner. In the former, bimetallic catalytic [4 + 2] cycloaddition worked well to provide a series of 6,6-benzannulated spiroketals, whereas the latter gave unprecedented nitrogen-containing 5,6-benzannulated spiroketals through catalytic [3 + 2] cycloaddition under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...