Highly functionalized cyclohexanone-monocyclic polyprenylated acylphloroglucinols from Hypericum perforatum induce leukemia cell apoptosis†
Abstract
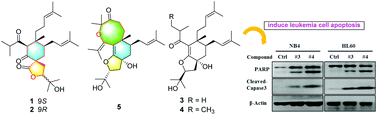
Spirohypolactones A (1) and B (2) and hyperhexanones C–E (3–5), five degraded cyclohexanone-monocyclic polyprenylated acylphloroglucinol (C-MPAP) derivatives were isolated from the stems and leaves of Hypericum perforatum. The structures of these compounds were established by extensive spectroscopic analyses and electronic circular dichroism (ECD) calculations. Compounds 1 and 2 are the first examples of naturally occurring C-MPAPs possessing 2-oxaspiro[4.5]decane spirocyclic skeletons. Compound 5 featured an unusual dodecahydro-4H-oxocino[4,3-g]benzofuran ring system. Notably, compounds 3 and 4 induce apoptosis in the acute myeloid leukemia cell lines NB4 and HL-60 by the activation of caspase-3, and degradation of PARP. The proposed biosynthetic pathways and biological activities are also discussed.


 Please wait while we load your content...
Please wait while we load your content...