Palladium-catalyzed regioselective C–H alkynylation of indoles with bromoalkynes in water†
Abstract
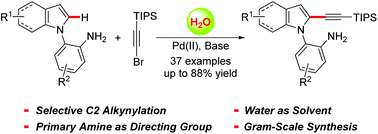
A highly regioselective alkynylation of indoles has been accomplished for the assembly of functionalized C2 alkynylindoles in moderate to good yields with a primary amine as the directing group. This protocol represents an efficient palladium-catalyzed C(sp2)–H activation/alkynylation using water/aqueous media as a sustainable solvent. Moreover, the scalability was demonstrated and further transformations of the alkynylating products were achieved, demonstrating the potential applications in synthetic and pharmaceutical chemistry. Preliminary mechanistic investigations suggest that the cleavage of the C2 C–H bond of indoles is likely to be the rate-determining step in this reaction.


 Please wait while we load your content...
Please wait while we load your content...