3,3-Diazidoenones – new types of highly reactive bis-azides. Preparation and synthetic transformations†
Abstract
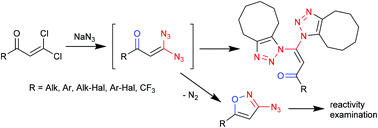
The interaction of geminal dichlorovinylketones with sodium azide was investigated by NMR and DFT calculations. It was found that the corresponding geminal 3,3-diazidoenones are the resultant species. These highly reactive compounds can be trapped with cyclooctyne to form bis-triazolyl substituted enones. In the absence of a trapping agent, 3,3-diazidoenones do cyclize into 3-azidoisoxazoles with the elimination of molecular nitrogen. The versatile reactivity of the resultant azidoisoxazoles was demonstrated in reactions with phosphorus(III) derivatives, Huisgen 1,3-dipolar cycloaddition and Dimroth cyclization to provide efficient access to a variety of valuable compounds.


 Please wait while we load your content...
Please wait while we load your content...