Synergistic promotion by intramolecular hydrogen bonding: a bi-functionally catalyzed cascade reaction for the synthesis of enantiopure chromenopyrrolidines†
Abstract
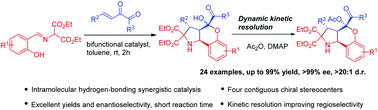
An effective organocatalytic asymmetric cascade reaction of o-hydroxy aromatic aldimines and β,γ-unsaturated-α-ketoesters was developed. Intramolecular hydrogen-bonding activation promoted the generation of fused ring frameworks which bear four contiguous stereocenters including one quaternary stereocenter in one-pot operation with excellent yields and stereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...