Efficient synthesis of tetrasubstituted 2,3-allenoates and preliminary studies on bioactivities†
Abstract
A general and highly efficient straightforward protocol for the preparation of tetrasubstituted 2,3-allenoates through a palladium-catalyzed coupling reaction of 3-alkoxycarbonyl propargylic carbonates and boronic acids with commercially available tri(o-tolyl)phosphine as a ligand has been developed. This mild reaction exhibited a wide substrate scope and functional group compatibility, providing a highly efficient strategy to access valuable tetrasubstituted 2,3-allenoate products. Several tetrasubstituted 2,3-allenoates obtained in this study exhibited potent anti-cancer and anti-diabetic activities.
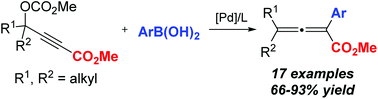


 Please wait while we load your content...
Please wait while we load your content...