Gold-catalyzed oxidative cycloalkenations of alkynes with quinoline N-oxides†
Abstract
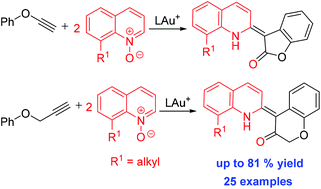
This work reports gold-catalyzed oxidative cycloalkenations of phenyl propargyl ethers and phenoxyalkynes with quinoline N-oxides to afford 4-alkylidenechroman-2-ones and 3-alkylidenebenzofuran-2-ones, respectively. This catalytic sequence involves one alkyne, one aryl nucleophile and two discrete quinoline oxides. We postulate a mechanism involving an initial formation of an α-oxo gold carbene that is attacked by a tethered arene to form a gold enolate, further reacting with a second quinoline N-oxide to complete the olefination process.


 Please wait while we load your content...
Please wait while we load your content...