Asymmetric synthesis of highly functionalized furanones via direct Michael reactions mediated by a bulky primary amine†
Abstract
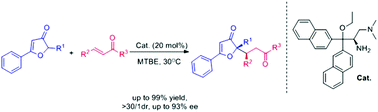
The asymmetric Michael reaction of 3(2H) furanones and α,β-unsaturated ketones was investigated, with the employment of a bulky chiral primary amine. The protocol afforded a series of substituted furanone derivatives in a highly diastereo- (>30 : 1 dr) and enantioselective (up to 93% ee) manner with generally good to excellent yields (up to 99%).


 Please wait while we load your content...
Please wait while we load your content...