Selective synthesis of aryl thioamides and aryl-α-ketoamides from α-oxocarboxylic acids and tetraalkylthiuram disulfides: an unexpected chemoselectivity from aryl sulfonyl chlorides†
Abstract
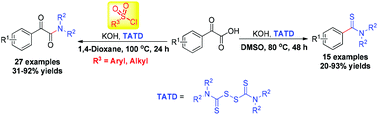
Aryl sulfonyl chloride-controlled generation of aryl thioamides and aromatic α-ketoamides through base-promoted decarboxylative functionalization of α-oxocarboxylic acids with tetraalkylthiuram disulfides has been established. The diverse formation of these two products could be controlled by the addition of tosyl chloride in different solvents. This protocol is compatible with a variety of electron-donating and electron-withdrawing functional groups and provides an attractive means to produce aryl thioamides and aromatic α-ketoamides with moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...