From glucose to enantiopure morpholino β-amino acid: a new tool for stabilizing γ-turns in peptides†
Abstract
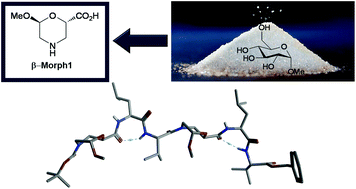
A new cyclic enantiopure β-amino acid, named β-Morph, containing the morpholino ring, was obtained in gram scale starting from an α-D-glucopyranose enantiopure material, focusing on the “environmental sustainability” concept. A series of ultrashort model peptides containing β-Morph were prepared. β-Morph was found to be able to induce an inverse γ-turn in Leu-Val containing sequences. The key element for the γ-turn formation might be the oxygen atom of the morpholino ring that could drive the spatial orientation of the NH of amino acid i + 1, through an unusual hydrogen bond. This datum was confirmed by QTAIM calculations. Interestingly, when two β-Morph-Leu-Val repeats are present in the peptide, two γ-turns can be formed, as supported by NMR experiments and aMD and H-REMD calculations.


 Please wait while we load your content...
Please wait while we load your content...