Understanding the Z selectivity of the metal-free intermolecular aminoarylation of alkynes: a DFT study†
Abstract
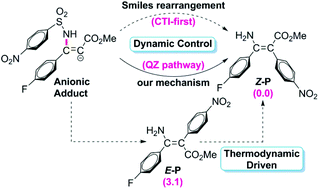
The transition metal-catalyzed hydro- or carboamination of alkynes usually yields cis enamines, indicating that the hydrogen or the unsaturated carbon group is located at the same side of the olefin bond with the amine group. Recently, Greaney and coworkers reported a metal-free intermolecular aminoarylation of alkynes, affording tetrafunctionalized enamines with the amine and aryl groups in trans geometry. The Smiles rearrangement was proposed to account for the aminoarylation mechanism, but the Z selectivity (i.e. trans enamine as the main product) is quite difficult to understand. In the present paper, the aminoarylation mechanism has been thoroughly studied and accordingly, the origin of the Z selectivity has been discussed in detail. The calculated results reveal that the Smiles rearrangement mechanism is able to occur under experimental conditions, but the novel mechanism, in which an alternative pathway is proposed for transformation of the anionic adduct, is more reasonable on account of the lower energy barrier and the more rational explanation for the roles of the additive K2CO3. The Z selectivity is determined by the inherent requirements of the dynamic preference of pathways (the CTI-first pathway in the Smiles rearrangement mechanism and the QZ pathway in the novel mechanism) that promise the Z-geometry product, rather than the conventional isomerization of the E-geometry product in situ with the assistance of K2CO3.


 Please wait while we load your content...
Please wait while we load your content...