Pd-Catalyzed carboannulation of γ,δ-alkenyl oximes: efficient access to 5-membered cyclic nitrones and dihydroazines†
Abstract
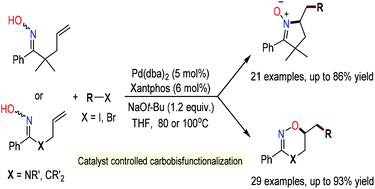
We report that the Pd0-catalyzed carboannulation of γ,δ-alkenyl oximes with aryl and vinyl halides offers a straightforward entry path to 5-membered cyclic nitrones or 6-membered cyclic oxime ethers, wherein substrate conformation can result in divergent reactivity, a phenomenon that likely arises from a shift in the mechanistic paradigm. The title transformation features a catalyst-controlled bond formation event that holds promise for the enantioselective synthesis of these important heterocycles.


 Please wait while we load your content...
Please wait while we load your content...