Silver-promoted selective fluorination of 2-aminopyrimidines: synthesis of 5-fluoro-2-aminopyrimidine derivatives†
Abstract
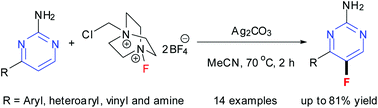
Selective fluorination of 4-substituted 2-aminopyrimidine derivatives with Selectfluor in the presence of Ag2CO3 is presented. The substrates are 4-aryl- or vinyl- or amine-substituted 2-aminopyrimidines. This method gives 4-substituted 5-fluoro-2-aminopyrimidines in fair to high yields with excellent regioselectivities. 5-Fluoro-4-(pyridin-3-yl)-2-aminopyrimidine was transformed into 5-fluoro-N-(2-methyl-5-nitrophenyl)-4-(pyridin-3-yl)pyrimidin-2-amine in the synthesis of fluorinated imatinib base.


 Please wait while we load your content...
Please wait while we load your content...