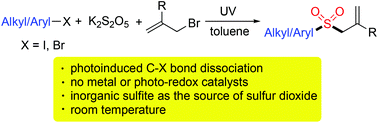
Photoinduced synthesis of allylic sulfones using potassium metabisulfite as the source of sulfur dioxide†
Abstract
Synthesis of allylic sulfones through a photoinduced three-component reaction of aryl/alkyl halides, potassium metabisulfite, and allylic bromides under ultraviolet irradiation at room temperature is developed. Diverse allylic sulfones are generated in moderate to good yields without the addition of any metals or photoredox catalysts. Different functional groups, including amino, ester, cyano, trifluoromethyl, and chloro are compatible under the conditions. Not only aryl halides but also alkyl halides are workable in the transformation. During this process, the natural abundant potassium metabisulfite is used as the source of sulfur dioxide. A plausible mechanism which involves the photoinduced C–X bond dissociation, sulfur dioxide fixation, and addition of the sulfonyl radical to allylic bromide is proposed.


 Please wait while we load your content...
Please wait while we load your content...