Stereoselective synthesis of a 9-O-sulfo Neu5Gc-capped O-linked oligosaccharide found on the sea urchin egg receptor†
Abstract
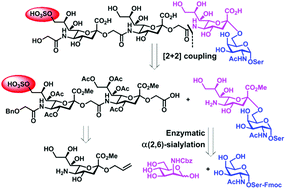
A straightforward synthesis of the α(2→5)-linked 9-O-sulfated Neu5Gc-capped O-linked tetrasaccharide (1) identified in the egg cell surface glycoproteins of the sea urchin Strongylocentrotus purpuratus receptor for sperm is reported. The construction of the α(2→5)Neu5Gc trimer by the formation of an amide linkage rather than a glycosidic bond avoids the requirement for α-stereoselective glycosylation. To highlight this amide bond formation strategy as a relatively facile method for synthesizing oligo-Neu5Gc containing O-linked glycans, its versatility is demonstrated by application to the coupling of SO3H-9Neu5Gcα(2→5)Neu5Gc glycolic acid and a sialyl-Tn-derived amine for achieving the target tetrasaccharide. This synthetic strategy may be implemented in the generation of other structurally similar O-sulfo Neu5Gc-capped α2→5-Oglycolyl-linked oligo-Neu5Gc chains, enabling additional biological and chemical investigations.


 Please wait while we load your content...
Please wait while we load your content...