Trifluoromethylation of α-diazoesters and α-diazoketones with fluoroform-derived CuCF3: synergistic effects of co-solvent and pyridine as a promoter†
Abstract
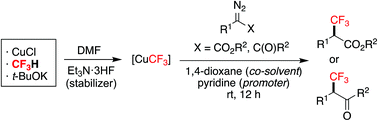
We herein describe a new protocol for copper-mediated trifluoromethylation of α-diazoesters and α-diazoketones, both using readily available fluoroform-derived CuCF3. The synergistic effect of using 1,4-dioxane as a co-solvent and pyridine as a promoter is revealed. Reaction conditions are mild and easy to handle, allowing the synthesis of a range of α-trifluoromethyl esters and ketones in one-step from α-diazo carbonyl compounds. The ultimate CF3 source is the inexpensive, nontoxic, industrial waste fluoroform.


 Please wait while we load your content...
Please wait while we load your content...