Unprecedented thiocarbamidation of nitroarenes: a facile one-pot route to unsymmetrical thioureas†
Abstract
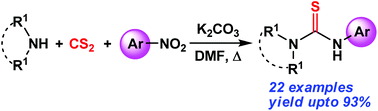
Aromatic nitro compounds undergo one-pot thiocarbamide functionalization upon reacting with in situ generated dithiocarbamate anions in the presence of K2CO3 and DMF solvent to produce unsymmetrical thiourea compounds in good yields. Various cyclic and acyclic 2° amines, 1° amines, and aromatic amines reacted with CS2 to form dithiocarbamate anions which combine with different nitroarenes resulting in the formation of thiourea compounds. The reaction is assumed to proceed through a nitrosobenzene intermediate.


 Please wait while we load your content...
Please wait while we load your content...