Carbon–oxygen-bridged hexacyclic non-fullerene acceptors with chlorinated end groups†
Abstract
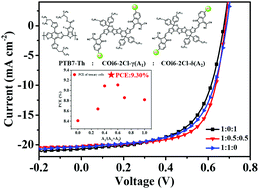
Three new chlorine substituted non-fullerene acceptors (COi6-2Cl-γ, COi6-2Cl-δ and COi6-2Cl-m) based on a highly electron-rich core, COi6, are designed and synthesized, showing ultra-narrow bandgaps as low as 1.31 eV. The oxygen-containing conjugated COi6 possesses a strong electron-donating capacity, resulting in strong intramolecular charge transfer and intermolecular π–π stacking at the derived acceptors. Three kinds of chlorine-substituted end groups have been chosen to react with the COi6 core in order to study the effect of the chlorine atom positioning on the performance of the final acceptor materials. The solar cells based on these three acceptor materials and a spectrally complementary polymer donor, PTB7-Th, have been systemically studied. It is noted that the mixed acceptor, COi6-2Cl-m, shows a relatively high power conversion efficiency (PCE) of 9.22%, which is better than that of the other two isomer-free acceptors. With detailed investigations on five different mixing ratios of ternary cells using two isomer-free small molecules as a co-acceptor (COi6-2Cl-γ : COi6-2Cl-δ), the highest PCE of 9.30% was obtained in ternary cells when the ratio of COi6-2Cl-γ and COi6-2Cl-δ was 1 : 1. From this observation, it is demonstrated that mixed materials in appropriate proportions are better than certain structure materials, COi6-2Cl-γ and COi6-2Cl-δ, in our system.


 Please wait while we load your content...
Please wait while we load your content...