Band structure engineering of boron–oxygen-based materials for efficient charge separation†
Abstract
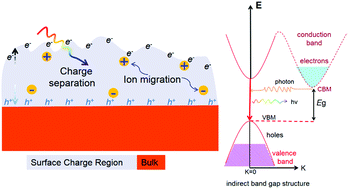
Efficient charge separation and suitable band alignment are critical for developing excellent photocatalysts. Boron–oxygen-based materials have been proven to be significant semiconductor photocatalysts to solve the energy crisis. Our results suggested that the separation of photogenerated carriers can be promoted by tuning the band gap of the materials from indirect to direct by the substitution of cations. Almost 87.8% and 71.5% of the total chloride contents were converted to Cl− anions after 20 minutes of UV-Vis light illumination for the indirect-band-gap material KBBO and the direct-band-gap material NBBO for 2,4-DCP degradation, respectively. The dechlorination efficiency of KBBO was approximately 5.3 times that of the commercial P25 TiO2 catalyst. The increased activity could be ascribed to the different locations of the photoinduced charges, making the charges have a long-term effect on the contaminants. By the KPFM method, we concluded that the materials with larger surface potential changes would behave higher photocatalytic activity. This contribution can provide a new approach to optimize the boron–oxygen-based materials for designing efficient photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...