High-pressure synthesis of highly oxidized Ba0.5Sr0.5Co0.8Fe0.2O3−δ cubic perovskite†
Abstract
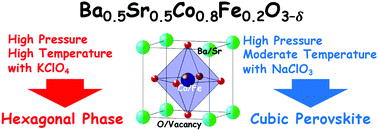
A cubic perovskite oxide Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) has been termed as a representative energy-conversion material. This compound includes intrinsic oxygen deficiency denoted as δ. We obtained a highly oxidized cubic perovskite BSCF by using high-pressure and moderate-temperature (8 GPa and 500 °C) conditions with NaClO3 as the oxidizing agent, although the hexagonal phase was obtained when treated at conventional high-pressure and high-temperature (8 GPa and 1000 °C) conditions with KClO4 as the oxidizing agent. The highly oxidized BSCF sample contained a small amount of oxygen deficiency (δ = 0.12) compared to the BSCF samples (δ = 0.38) synthesized in typical conditions, thus proposing an alternative route to obtain the metastable phase of highly oxidized perovskite. The electric and magnetic measurements revealed that the metallic phase was predominant for the highly oxidized sample (δ = 0.12) in contrast to the non-metallic properties for the conventional oxygen-deficient sample (δ = 0.38). The catalytic activity for oxygen evolution reaction (OER) was evaluated within a wide range of oxygen content from heavily oxygen-deficient (δ = 0.51) to conventional (δ = 0.38) to highly oxidized (δ = 0.12) samples. Both the highly oxidized and heavily oxygen-deficient samples were inferior to the conventional one in OER catalytic activity, indicating that an adequate amount of oxygen deficiency is favorable for the activation of OER by BSCF.


 Please wait while we load your content...
Please wait while we load your content...