Electrodepositing Pd on NiFe layered double hydroxide for improved water electrolysis
Abstract
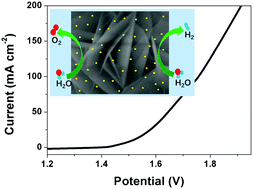
Exploring electrocatalysts with advanced bifunctionality for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is significantly important and highly required to efficiently achieve energy conversion via water splitting. Herein, ultrafine Pd nanoparticles are electrodeposited upon hydrothermally grown NiFe layered double hydroxide (NiFe LDH) on nickel foam for pursuing improved electrocatalysis bifunctionality. The introduced Pd induces more active sites, strong electric interaction, and enhanced charge transfer, thus leading to substantially improved catalytic activity for water electrolysis. The optimal Pd-NiFe LDH exhibits impressive catalytic activity, affording a current density of 10 mA cm−2 at low overpotentials of 156 mV for the OER and 130 mV for the HER, respectively. The two-electrode electrolyzer assembled with Pd-NiFe LDH achieves an ultralow cell potential of 1.514 V at 10 mA cm−2 for overall water splitting, superior to those of most of the previous bifunctional electrocatalysts in alkaline media. This study may offer a promising solution to simultaneously improve the intrinsic activity, site density, and charge transfer of transition metal electrocatalysts with the help of trace amounts of noble metal.
- This article is part of the themed collection: 2019 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...