A highly selective fluorescent probe for real-time imaging of bacterial NAT2 and high-throughput screening of natural inhibitors for tuberculosis therapy†
Abstract
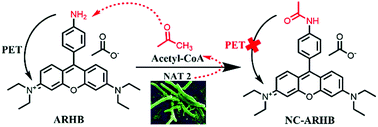
N-Acetyltransferase 2 (NAT2) is a very important biologically functioning enzyme which plays a key role in the synthesis of cell walls in slowly growing mycobacteria. Thus, it has been widely considered to be a drug-target for anti-mycobacterial therapy. Herein, based on its catalytic characteristics, ARHB was developed for the real-time monitoring and assessment of the activity of NAT2 in different bacteria such as Mycobacterium, Staphylococcus aureus, Lactobacillus, and Pseudomonas aeruginosa. Moreover, using the highly sensitive probe ARHB, a potent natural inhibitor (KSB-4) of NAT2 was found which could effectively inhibit Mycobacterium tuberculosis H37Ra for the first time. Furthermore, the bioactivity of KSB-4 for treating tuberculosis was also evaluated in M. tuberculosis H37Ra, showing a minimum inhibitory concentration (MIC) of 25 μM. After treatment with KSB-4, M. tuberculosis H37Ra grew to be much shorter and its cell wall lysis was observed in scanning electron micrographs. All of the results fully demonstrated that ARHB could serve as a promising molecular tool for the rapid and real-time detection of NAT2 activity in a complex system, providing a novel high-throughput screening method for the discovery of new NAT2 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...