La-Doped ZnWO4 nanorods with enhanced photocatalytic activity for NO removal: effects of La doping and oxygen vacancies†
Abstract
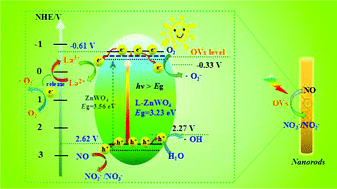
La3+-Doped ZnWO4 nanorods were prepared via a hydrothermal method for the photocatalytic NO removal under simulated solar light irradiation. It was revealed by photocatalytic experiments that the 0.6% La3+-doped ZnWO4 nanorods exhibited the best photocatalytic activities for NO removal (46%) in comparison with other samples. The enhanced photocatalytic activity could be attributed to La3+ ion doping, which creates an abundance of oxygen vacancies (OVs) as confirmed by the electron paramagnetic response (EPR). The optical response ability was promoted by La3+ ion doping and the construction of OVs according to the results of UV-vis spectra. Density functional theory (DFT) calculations were further conducted, revealing that the introduction of La3+ ions and high concentrations of OVs can narrow the band gap of ZnWO4. The e− and ˙O2− radical species are found to play critical roles in the oxidative removal process via the scavenger experiments and the DMPO-ESR measurements. Furthermore, the possible photocatalytic reaction mechanism was also proposed based on the products formed during the photocatalytic reaction, which were traced by in situ Fourier transform infrared spectroscopy (in situ FTIR). Overall, the findings in this work can provide inspiration for the design and synthesis of rare earth ion-doped and OV-rich photocatalysts with highly efficient photocatalytic NO removal.


 Please wait while we load your content...
Please wait while we load your content...