Optimized Co2+(Td)–O–Fe3+(Oh) electronic states in a spinel electrocatalyst for highly efficient oxygen evolution reaction performance†
Abstract
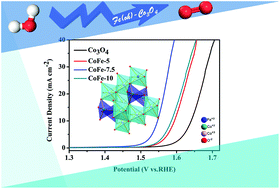
Electronic configuration is crucial for enhancing the catalytic activities of spinels for the oxygen evolution reaction (OER). However, controlling the electronic spin state of materials is still a challenge. In this work, we synthesized Fe-doped meso-Co3O4via a nanocasting method. The merit of our method lies in high spin state Fe3+ (t2g3eg2) being controllably introduced into an octahedral site to regulate the valence states and configure the eg electron of Co3+. The introduced Fe3+ prefers to occupy octahedral sites due to its lower formation energy. Then, Fe3+ doping enlarges the Co3+–O distance and decreases the lattice symmetry, leading to the splitting of the d-orbital in Co3+. Our density functional theory (DFT) calculations reveal that spin state optimized Co3+(Oh) acts preferentially as an active site. Furthermore, CoFe-7.5 (Co2.775Fe0.225O4), with its maximum Fe3+(Oh) content, exhibits the best OER activity. Our work indicates that the introduction of Fe3+ enables an improvement in the electrocatalytic performance of Co3O4 by regulating the spin state of Co3+.


 Please wait while we load your content...
Please wait while we load your content...