Exposing the photocorrosion mechanism and control strategies of a CuO photocathode†
Abstract
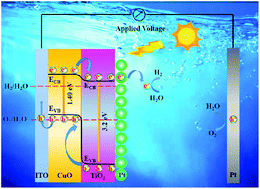
Photocorrosion is regarded as an important limitation of cupric oxide (CuO) for application in photocatalysis and photoelectrocatalysis. In this paper, we firstly explore the photocorrosion mechanism of a CuO photocathode in the photoelectrocatalysis field and reveal that self-reduction of CuO caused by photogenerated electrons is a prime factor for the photocorrosion of the CuO photocathode. Aiming to solve this issue, TiO2 is introduced on the surface of CuO to form a suitable gradient band level, which is conducive to the fast export of photo-induced electrons in the bulk of CuO. Besides, a Pt cocatalyst as an electron capture layer (ECL) is selected to load on top of the CuO/TiO2 photoelectrode, further promoting the transport of electrons, thereby improving the photocorrosion stability of the CuO photocathode. Under the synergistic effect of TiO2 and Pt, the CuO/TiO2/Pt photocathode in photoelectrochemical water splitting exhibits a photocurrent density of −0.75 mA cm−2 at −0.55 V vs. Ag/AgCl and a photocorrosion stability of 84% after the stability test for 2 h. This work not only reveals the photocorrosion mechanism of the CuO photocathode, but also offers a new method for improving the photocorrosion stability of photocathodes.


 Please wait while we load your content...
Please wait while we load your content...