Electrochemical properties of chromium oxyfluoride CrO2−xFx with 0 ≤ x ≤ 0.3†
Abstract
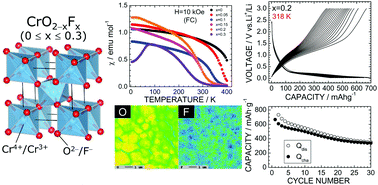
To overcome the limitations of graphite as a negative electrode material for lithium-ion batteries (LIBs), transition metal oxyfluorides are under active development. In this study, chromium oxyfluorides CrO2−xFx with 0 ≤ x ≤ 0.3 were synthesized under a high-pressure/high-temperature (HP/HT) environment, and their electrochemical properties were examined in a nonaqueous lithium cell. The HP/HT-treated CrO2 maintained a rutile structure and exhibited a rechargeable capacity (Qrecha) of over 400 mA h g−1 at 298 K. The replacement of O2− ions with F− ions in CrO2 was confirmed by linear changes in the tetragonal lattice parameters, weaker ferromagnetic interactions between Cr4+ ions, and elemental mappings of F− ions. The Qrecha values of the x > 0 samples at 298 K decreased to 150–300 mA h g−1 because of low electric conductivity in CrO2−xFx. However, the Qrecha values at 318 K increased to 600–700 mA h g−1, and the cycle performance over 30 cycles was better than that of the HP/HT-treated CrO2 sample with no F− substitution. Hence, CrO2−xFx was found to be a promising negative electrode material for LIBs, although its cycle stability should be further improved.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...