Solvent switching smart metal–organic framework as a catalyst of reduction and condensation†
Abstract
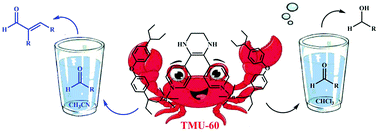
The organization of a Zn-based metal–organic framework (MOF) as the first solvent switching catalyst has been achieved via in situ ligand incorporation. The catalytic mechanism of TMU-60 (Zn (OBA) (L*)) can be changed by a special solvent which led to reversible changes in its structure. The switching process leads to the production of two completely different products from the same raw material. The local reversible dynamic conversion of the ligand functionality from amine to imine on impact of chloroform led to a noticeable hydrogen generation/selective aldehyde reduction to the corresponding alcohol in the absence of a reductant. The catalyst recovery process was easily and quickly performed by immersing the framework in DMF. 1H NMR spectroscopy, IR spectroscopy and the results of GC and GC mass confirm the switching process. However, in the presence of acetonitrile, a different catalytic mechanism, i.e., the aldol condensation reaction, occurred by TMU-60 in a short period at ambient temperature. This study represents a simple approach for the construction of a switchable MOF, and these smart systems may provide a unique MOF platform for many applications, particularly as catalysts, in the future.


 Please wait while we load your content...
Please wait while we load your content...