Design of Mo-doped cobalt sulfide hollow nanocages from zeolitic imidazolate frameworks as advanced electrodes for supercapacitors†
Abstract
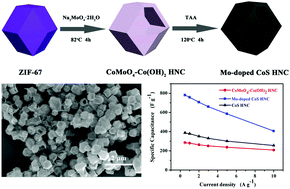
Construction of ternary metal sulfides with hollow nanostructures as energy storage materials is very promising yet challenging. Herein, we report a Mo-doped cobalt sulfide with a unique hollow nanocage structure (Mo-doped CoS HNC) by using ZIF-67 as a single sacrificial template through a dissolution–regrowth process in the presence of Na2MoO4 with an additional sulfurization process. The obtained Mo-doped CoS HNC exhibits an enhanced specific capacitance (781.0 F g−1 at 0.5 A g−1) compared with the control CoS HNC (387.1 F g−1) and CoMoO4–Co(OH)2 HNC (285.1 F g−1). Furthermore, it also shows a superior rate capacity of 52.0% under a 20-fold increase of current density (10 A g−1). An asymmetric supercapacitor (ASC) device assembled by using the Mo-doped CoS HNC as a positive electrode and activated carbon (AC) as a negative electrode displays a high energy density of 27.7 W h kg−1 at a power density of 799.9 W kg−1 with excellent cycling stability, showing that 88.0% of the initial capacitance is maintained after 10 000 cycles. The excellent electrochemical performance arises from the unique ternary metal sulfide hollow nanocage structure having a large surface area, facile diffusion of ions, good conductivity, and rich redox reactions as well as the synergistic effect between Mo and Co ions.


 Please wait while we load your content...
Please wait while we load your content...