Photoluminescence of Ag(i) complexes with a square-planar coordination geometry: the first observation†
Abstract
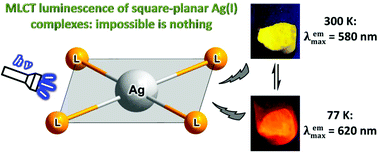
The first observation of luminescence from Ag(I) complexes with a square-planar geometry of the metal is reported. [Ag(Py3PO)2]ClO4 and [Ag(Py3PO)2]OTf – first examples of the emissive square-planar Ag(I) complexes – have been synthesized by the reaction of tris(2-pyridyl)phosphine oxide (Py3PO) with Ag(I) salts. Both complexes exhibit pronounced photoluminescence in the solid state at ambient temperature with nano- and microsecond lifetime components. Remarkably, the luminescence of the complexes has a reversible thermochromic behavior, changing their emission color from yellow (λemmax ≈ 585 nm) at room temperature to red-orange (λemmax = 620 nm) at 77 K. The spectroscopic and computational results reveal that the emission of the title compounds can be assigned to the 3MLCT phosphorescence with admixture of the 1MLCT fluorescence.


 Please wait while we load your content...
Please wait while we load your content...