Supercapacitive properties of amorphous MoS3 and crystalline MoS2 nanosheets in an organic electrolyte†
Abstract
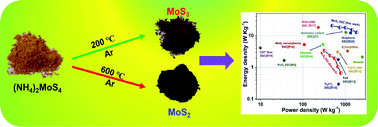
Molybdenum sulfide materials receive high attention as high-performance electrodes for electrochemical energy storage devices. In this study, we investigate the electrochemical energy storage properties of amorphous MoS3 and crystalline MoS2 materials (prepared via thermal decomposition of ammonium tetrathiomolybdate) using an organic liquid electrolyte. Physicochemical characterization using X-ray diffraction pattern and laser Raman analysis confirms the formation of amorphous MoS3 and crystalline MoS2, respectively. The energy storage properties of MoS3 and MoS2 based symmetric supercapacitor devices were comparatively studied using cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge–discharge analysis. The cyclic voltammetry analysis reveals the mechanism of charge storage in MoS3 and MoS2 is due to the ion-intercalation/de-intercalation pseudocapacitance. Electrochemical impedance spectroscopy studies reveal the better capacitance and charge-transfer nature of the crystalline MoS2 symmetric supercapacitor compared to that of the amorphous MoS3 symmetric supercapacitor. The charge–discharge analysis suggests that the MoS2 symmetric supercapacitor device possesses better electrochemical energy storage properties with a high specific capacity of 20.81 mA h g−1 (24.98 F g−1) and energy density of about 20.69 W h kg−1 with the excellent cyclic stability of about 2000 cycles. The experimental results suggest that the crystalline MoS2 sheets might be a better choice than amorphous MoS3 as an electrode material for supercapacitors using an organic liquid electrolyte.


 Please wait while we load your content...
Please wait while we load your content...