In Operando analysis of the charge storage mechanism in a conversion ZnCo2O4 anode and the application in flexible Li-ion batteries†
Abstract
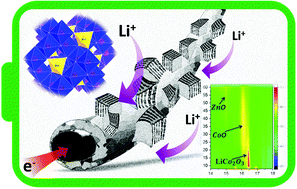
As a conversion-type electrode material, ZnCo2O4 (ZCO) is intensively researched due to its attractive high specific capacity. Much effort to study ZCO supported on a conductive matrix has been successful to overcome the inherent drawbacks of low conductivity and dramatic volume variation during the (de)lithiation process. Despite many reported studies, the lithiation storage mechanism in the ZCO electrode is not yet clearly elucidated. In this work, in operando synchrotron radiation diffraction and in operando X-ray absorption spectroscopy are used to study the lithium storage mechanism in the ZCO material. The initial conversion process of ZnCo2O4, involving multiple reactions based on intercalation, conversion and alloying is deeply elucidated. During the 1st lithiation intermediate phases such as LiCo2O3, CoO and ZnO are formed. On the other hand, upon delithiation, the conversion to ZnO and CoO (and not to the pristine ZnCo2O4) occurs. This is different from the previous conclusion, which claims that Co3O4 forms after the initial delithiation. Furthermore, a binder-free ZnCo2O4/carbon cloth composite electrode is also prepared, which exhibits higher rate performance and capacity retention, compared to the bare ZCO electrode.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...