A NiII complex of the tetradentate salen ligand H2LNH2 comprising an anchoring –NH2 group: synthesis, characterization and electrocatalytic CO2 reduction to alcohols†
Abstract
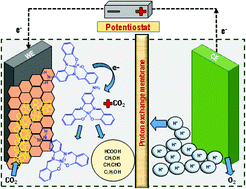
A new salen ligand with an anchoring free amine functional group in the ligand backbone and the corresponding NiII complex [NiIILNH2] (1) were synthesized and characterized. The complex effectively reduced CO2 to C1 (HCOOH, CH3OH) and C2 (C2H5OH) products in a simple H-type reactor upon immobilization on the graphite electrode. Initially HCOOH was formed which was further reduced to CH3OH and C2H5OH in a 6e− and 12e− transfer pathway. An overall current efficiency of 49% was recorded for the formation of alcohols at −1.80 V vs. Ag/AgCl in aqueous media (ERC time 1 h, pH 7.0 and KHCO3 0.5 M). Thus, the common limitation of the formation of deeply reduced C2 products has been overcome. The effect of the anchoring free amine group was further established by examining the CO2 reduction efficiency of the similar NiII complex without having the tethered –NH2 group. The lack of alcohol production for this case highlighted the importance of the anchoring group with the electrodes during electrochemical reduction.


 Please wait while we load your content...
Please wait while we load your content...