Ethylene glycol-assisted fabrication and superb adsorption capacity of hierarchical porous flower-like magnesium oxide microspheres for phosphate
Abstract
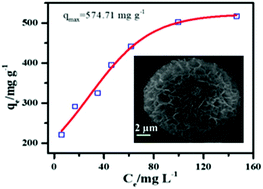
A series of hierarchical flower-like magnesium oxide (MgO) microspheres were prepared with an ethylene glycol (EG)-assisted route at room temperature using ammonia as a precipitating agent. Effects of the ethylene glycol (EG) ratio on the structure, morphology and pore properties were carefully investigated. The hierarchical porous MgO microsphere exhibited a surface area of 75 m2 g−1 and total pore area of 47.37 m2 g−1 at the highest ratio of EG (EG/Mg2+ = 10) in the reaction system. The prepared MgO microspheres exhibited an outstanding removal capacity of 574.71 mg g−1 for phosphate following the pseudo second order kinetic model (R2 = 0.99) and Langmuir isotherm model with the endothermic nature of phosphate adsorption which resulted from the high surface area and suitable pore size. Both the isothermal parameter RL between 0 and 1 and the negative the Gibbs free energy value suggested that phosphate adsorption on a MgO microsphere was a favorable process. Undoubtedly, this template-free mild synthesis method effectively promotes wide practical applications and mass scale production of porous MgO microsphere adsorption material.


 Please wait while we load your content...
Please wait while we load your content...