Stellerite-seeded facile synthesis of zeolite heulandite with exceptional aqueous Cd2+ capture performance†
Abstract
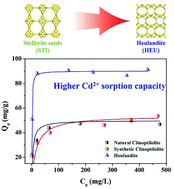
Clinoptilolite (HEU) mineral is by far the most common natural zeolite; it has been described as an excellent adsorbent for heavy metal ions and radioactive 137Cs+. Heulandite, which possesses the same framework topology as clinoptilolite, has a lower Si/Al ratio than clinoptilolite. Thus, superior performance is expected for heulandite in harmful ion removal due to its higher cation exchange capacity. However, it has proved to be a great challenge to synthesize heulandite because of the harsh synthesis conditions and poor reproducibility of the reported synthesis methods. Herein, heulandite was facilely obtained via hydrothermal synthesis using natural stellerite (STI) zeolite as a seed. This is the first time that natural stellerite zeolite has been used as a seed; however, it affords a different zeolite. The present results reveal that the phase selectivity of heulandite is sensitive to its crystallization conditions, including the SiO2/Al2O3 and Na2O/SiO2 ratios of the initial gel, seed content, and heating temperature. The pure heulandite can be readily reproduced in a narrow crystallization field. The as-synthesized heulandite shows much higher Cd2+ sorption capacity than natural or synthesized clinoptilolite. Compared to the Na+ form heulandite, the H+ form heulandite shows much better thermal stability; thus, heulandite has potential applications in catalysis and gas separation.


 Please wait while we load your content...
Please wait while we load your content...