Ultrasmall MoP encapsulated in nitrogen-doped carbon hybrid frameworks for highly efficient hydrogen evolution reaction in both acid and alkaline solutions†
Abstract
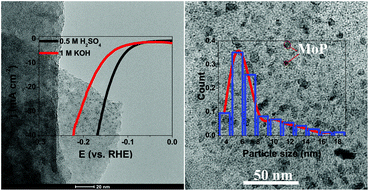
Improving the electrocatalytic performance of molybdenum phosphide-based (MoP) electrocatalysts through structure design has become a feasible method to use MoP to supersede noble metallic electrocatalysts for the hydrogen evolution reaction (HER). Herein, we demonstrated a two-step calcining strategy to synthesize a new excellent HER catalyst consisting of MoP nanoparticles loaded on a nitrogen-doped carbon hybrid framework substrate (MoP@NCF), for which acetone was used as the carbon source of the MoP-based electrocatalysts for the first time. The MoP@NCF exhibits an outstanding electrocatalytic performance for the HER with a low overpotential (only 121.8 mV in acid and 129.5 mV in alkaline electrolytes, respectively) at a current density of 10 mA cm−2, which is ascribed to the unique porous nanostructure with an enormous special surface area of 247.43 m2 g−1, and the ultrasmall size and homogeneous distribution of the MoP nanoparticles. Our work provides a rational method to reinforce the HER activity of molybdenum phosphide through structure design, and the therein useful strategy has the possibility of being applied to a range of other metal-based electrocatalyst materials.


 Please wait while we load your content...
Please wait while we load your content...