High-valence-state manganate(v) Ba3Mn2O8 as an efficient anode of a proton-conducting solid oxide steam electrolyzer†
Abstract
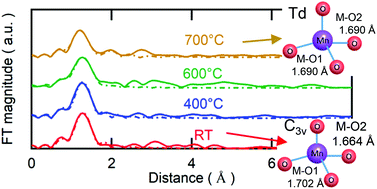
Herein, high-valence-state Mn(V) oxide, barium manganate(V) (Ba3(MnO4)2), is examined as an anode electrocatalyst of a H+-conducting solid oxide steam electrolysis cell (H-SOEC). Ba3(MnO4)2 comprises C3v-symmetric MnO43− oxo-anions with three long Mn–O bonds and one short Mn–O bond at room temperature. Ba3(MnO4)2 caused a conductivity jump by one order of magnitude at approximately 600 °C owing to the antiferromagnetic/paramagnetic phase transition, accompanied by a shape change of the tetrahedral MnO43− anions from C3v to Td symmetry, as confirmed by the electrical conductivity measurements and the extended X-ray absorption fine structure at an elevated temperature. Hence, the Ba3(MnO4)2 base anode of the H-SOEC exhibited improved performance, with anode polarization resistances being lower than those of Sm0.5Sr0.5CoO3, a well-known H-SOEC anode material. Impedance analysis in terms of oxygen and water partial pressure revealed that the superior performance of the Ba3(MnO4)2 base anode can be attributed to the extended reaction area. Since abundant unoccupied 3d states of the high-valence-state Mn5+ cations are favorable for charge transfer interactions with water electron donors, thereby facilitating water adsorption, the oxygen evolution reaction could occur directly over the electrode surface, and thus the reaction sites were not limited to the gas–electrode–electrolyte triple phase boundary.


 Please wait while we load your content...
Please wait while we load your content...