Copper–nitroxide based breathing crystals: a unified mechanism of gradual magnetostructural transition supported by quantum chemistry calculations†‡
Abstract
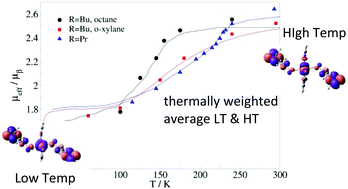
The molecular magnets Cu(hfac)2LR based on copper(II) and pyrazolyl-substituted nitronyl nitroxide radicals LR exhibit thermally and optically-induced magnetostructural transitions, similar to the spin-crossover and light-induced excited spin state trapping phenomena. The mechanism of the gradual change in the magnetic moment in Cu(hfac)2LR remains unclear. Herewith, we report a detailed study of this mechanism at the molecular level based on DDCI and periodic DFT+U calculations. Three representative members of the Cu(hfac)2LR family have been selected, with different substituents (R = Pr, Bu) and solvents (octane, o-xylane, without solvent). Our results indicate that the magnetostructural transition can be related to the coexistence of two limit structures, the low temperature (LT) phase with a strong coupling between the Cu(II) and nitronyl nitroxide spins and the high temperature (HT) phase, where the spins are weakly coupled. In this scenario, the gradual change in the magnetic moment with temperature just reflects the thermally weighted ratio of the two limit LT and HT phases. Our findings support the changes observed in the variable temperature-FTIR spectra of Cu(hfac)2LPr, manifested by the increase/decrease of certain vibrational bands with temperature and suggest a unified mechanism governing the gradual magnetic anomalies of the Cu(hfac)2LR complexes.


 Please wait while we load your content...
Please wait while we load your content...