An imidazole derivative-based chemodosimeter for Zn2+ and Cu2+ ions through “ON–OFF–ON” switching with intracellular Zn2+ detection†
Abstract
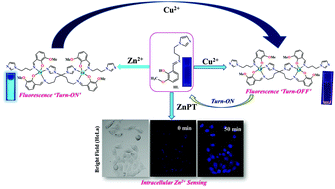
A new fluorescent receptor HL was synthesized by an easy condensation reaction of ortho-vanillin and 1-(3-aminopropyl) imidazole. The probe HL was found to be highly selective and sensitive towards Zn2+/Cu2+ ions in the presence of a wide range of metal cations. The interaction of HL with Zn2+ ions showed a distinct fluorescence enhancement (turn-on) at 470 nm. Moreover, after the subsequent addition of Cu2+ ions into the same solution, a fluorescence ‘turn-off’ phenomenon was observed. The sensing ability of the chemodosimeter HL towards Zn2+ was confirmed by fluorescence, UV-Vis and 1H NMR titration analysis. The binding mode of HL towards Zn2+ and Cu2+ was authenticated by single crystal X-ray analysis, which divulged the formation of dinuclear complexes 1 and 2. Furthermore, we performed NOESY and COSY studies of complexes 1 and 2 to detect their shapes and interlocking units. Moreover, the cytotoxicity of HL and 1 towards HeLa cancer cells was studied, and both were found to be non-cytotoxic in nature. This prompted us to explore the intracellular fluorescence turn-on sensing activity of HL with Zn2+ ions. The intracellular sensing behavior of HL towards Zn2+ was confirmed towards in two cancer cell lines viz., HeLa and DU-145 cell lines. The limit of detection (LOD) for Zn2+ and Cu2+ sensing was found to be 31.044 nM and 11.64 nM, respectively.


 Please wait while we load your content...
Please wait while we load your content...