Structural and thermodynamic aspects of water–carbonate exchange equilibrium for MIII/IV–EDTA–carbonate systems†
Abstract
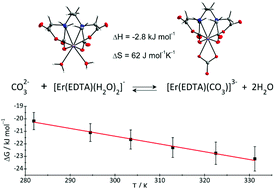
The results of a thermodynamic description of ternary M–EDTA–carbonate (where M = Er(III), Th(IV)) complex formation are presented. The crystal structures of the novel [C(NH2)3]3[Er(EDTA)(CO3)]·H2O (I) and [C(NH2)3]4[Th(EDTA)(CO3)2]·5H2O (II) compounds were determined. The structural and UV-vis-NIR spectroscopic results of crystal I served as the model data to analyze the stoichiometry and stability of the Er(III)–EDTA–carbonate system in aqueous solutions. The formation of the [Er(EDTA)(CO3)]3– complex in solution under different conditions was examined by complementary techniques including temperature dependent UV-vis-NIR and NMR spectroscopy as well as potentiometry. It was established that the affinity of the carbonate for the Er(III)–EDTA chelate is strongly pH dependent. Thus reaction (A) [Er(EDTA)(H2O)2]− + CO32– ⇄ [Er(EDTA)(CO3)]3– + 2H2O is more favoured at near neutral pH, while reaction (B) [Er(EDTA)(OH)(H2O)2]2– + CO32– ⇄ [Er(EDTA)(CO3)]3– + 2H2O + OH− occurs under more basic conditions. The log β values were found to be 3.66 ± 0.07 and 0.20 ± 0.12 for reactions (A) and (B), respectively. The temperature dependence of log β allowed the determination of the enthalpy and entropy changes of both reactions for the first time (ΔH(A) = −2.8 ± 0.8 kJ mol−1, ΔS(A) = 62 ± 3 J mol−1 K−1 and ΔH(B) = 28.1 ± 4.6 kJ mol−1, ΔS(B) = 92 ± 15 J mol−1 K−1). These data indicate that the carbonate anion more readily displaces H2O than the OH− ligand. The obtained results are important not only from the point of view of environmental lanthanide and actinide mobility, but also for designing MRI contrast agents.


 Please wait while we load your content...
Please wait while we load your content...