Synthesis of hyperbranched polyolefins and polyethylenes via ADMET of monomers bearing non-selective olefins†
Abstract
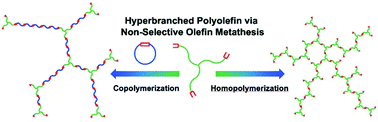
Polymerizations of olefinic monomers bearing 3-terminal olefins were achieved via the non-selective olefin metathesis for the synthesis of hyperbranched polyolefins. Three tri-functional monomers with 1, 2, and 6 carbon atoms between the central tertiary carbon atom and the terminal olefins were synthesized and polymerized with Grubbs’ first-generation catalyst (G1) and Grubbs’ third-generation catalyst (G3). NMR analysis was performed to investigate the kinetics and the related mechanisms for different monomers/catalysts. Our study revealed that two types of reactions, intramolecular ring-closing metathesis (the RCM route) and the intermolecular cross-metathesis (the ADMET route), participated in the polymerization. By tuning the monomer, catalyst, and concentration, the reaction could be driven in the direction of the ADMET route to obtain hyperbranched polymers. The resulting polymers could be hydrogenated to afford hyperbranched polyethylenes with well-defined local branching architectures. We also studied the copolymerization of the designed monomers with cis-cyclooctene (COE) for the synthesis of hyperbranched polyolefins and polyethylenes with long branches. Our work could provide a new solution for the construction of hyperbranched polyolefins and polyethylenes with well-defined branching architectures.


 Please wait while we load your content...
Please wait while we load your content...