Melamine-mediated supramolecular assembly of nucleobase-modified poly(l-lysine)†
Abstract
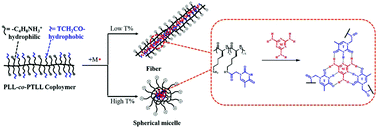
Polypeptides serve as a bridge between natural macromolecules and synthetic polymers, leading to the development of biocompatible and biodegradable materials with diverse and complex structures. Here, nucleobase modified polylysine (PLL-co-PTLL) was synthesized via post-polymerization modification of polylysine with thymine (T). Then, melamine (M) with three thymine-like faces was taken as a molecular chaperone analogue to drive the self-assembly of PLL-co-PTLL. It was observed that 7M–1T (M : T = 7 : 1) was the appropriate molar ratio for the pronounced self-assembly, resulting in regular and uniform fibers with an extremely high aspect ratio when the substitution degree of T was relatively low (around 27%). Furthermore, the morphological transformation from fibrous to spherical could be achieved by simply increasing the substitution degree of T. We proposed that melamine and thymine could assemble into a tetrameric structure, which enabled the accumulation of polypeptide chains in a cooperative nucleation-growth polymerization mechanism. Therefore, multiple H-bonding faces created between designable substitution polypeptides and complementary molecules would promote synergistic and hierarchical self-assembly processes.


 Please wait while we load your content...
Please wait while we load your content...