Significant improvement of photocatalytic hydrogen evolution of diketopyrrolopyrrole-based donor–acceptor conjugated polymers through side-chain engineering†
Abstract
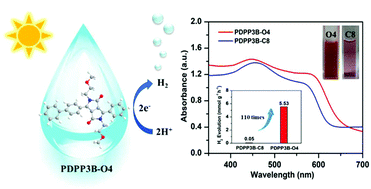
Donor–acceptor (D–A) type conjugated organic polymers exhibited great potential for photocatalytic hydrogen evolution due to their diverse synthetic approaches, tunable energy band, and electronic structure. But the poor dispersion of most conjugated organic polymers limited their photocatalytic performance. Herein, we designed and developed a series of D–A type conjugated organic polymers with benzene as the donor and diketopyrrolopyrrole (DPP) with different side chains on N sites as the acceptor. We changed the length of the side chain and further introduced oxygen atoms on side chains. As a result, the hydrogen evolution rate (HER) of PDPP3B-O4 with a short butoxy chain as the side chain was 5.53 mmol h−1 g−1 with 1 wt% Pt loading (λ > 400 nm), which increased 110 times compared to PDPP3B-C8 with a long octyl chain. All polymers showed outstanding photocatalytic stability. Notably, an apparent quantum yield (AQY) of 5.7% at 450 nm was achieved by PDPP3B-O4, and even a still high AQY of 1.13% at 600 nm was obtained due to its excellent light capture capability. PDPP3B-O4 showed a superior photocatalytic performance because of the wider absorption spectrum and better wettability via side chain engineering.


 Please wait while we load your content...
Please wait while we load your content...