Polybenzimidazole co-polymers: their synthesis, morphology and high temperature fuel cell membrane properties†
Abstract
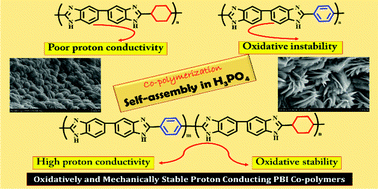
Polybenzimidazole (PBI) random co-polymers containing alicyclic and aromatic backbones were synthesized using two different dicarboxylic acids (viz., cyclohexane dicarboxylic acid and terephthalic acid) by varying their molar ratios. The synthesized co-polymers were characterized by inherent viscosity (IV) measurements, Fourier transform infrared spectroscopy (FTIR), 1H nuclear magnetic resonance (1H NMR) spectroscopy, X-ray diffraction (XRD) and thermo-gravimetric analysis (TGA). The co-polymer composition was determined by 1H NMR spectroscopy. The cyclohexyl based PBI possessed a lower proton conductivity (114 mS cm−1) than terephthalic acid based PBI (220 mS cm−1). The aromatic PBI had a high tensile modulus of 11 GPa, whereas the modulus of cyclohexyl PBI was only 2 GPa. By suitably selecting the monomer concentration, the co-polymer properties can be altered (both proton conductivity and mechanical properties). Among different co-polymers, one synthesized using 30 mol% cyclohexane dicarboxylic acid and 70 mol% terephthalic acid exhibited good elongation (8%) and modulus (10.5 GPa) values and improved proton conductivity (242 mS cm−1). In the doped condition, the co-polymer registered an elongation of 52% and a tensile modulus of 170 MPa. The high conductivity of this composition is attributed to the presence of ordered domains (shown by field emission scanning electron microscopy) present in the co-polymer in the doped condition. The co-polymers are thermally stable and the thermal stability increased with an increase in the aromatic content. Thus, alicyclic–aromatic co-polymerization is a viable technique to prepare high-temperature proton exchange membranes.
- This article is part of the themed collection: Polymer Chemistry Most Popular 2020


 Please wait while we load your content...
Please wait while we load your content...