A solvent-less green synthetic route toward a sustainable bio-based elastomer: design, synthesis, and characterization of poly(dibutyl itaconate-co-butadiene)†
Abstract
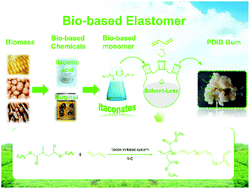
A sustainable poly(dibutyl itaconate-co-butadiene) (PDIB) elastomer with curable double bonds was synthesized by environmentally benign emulsion polymerization of bio-based dibutyl itaconate with butadiene. The microstructure of the PDIB elastomer was confirmed by FTIR and NMR. The resulting copolymers had predominately trans- and vinyl-polybutadiene in their chains, a molecular weight from 236 000 to 392 000 Da, and a glass transition temperature from −42 to −72 °C, depending on the feed weight percentage of butadiene. The reactivity ratios of dibutyl itaconate and butadiene determined by the classical Fineman–Ross method and Kelen–Tüdös one indicated a non-ideal copolymerization behavior with an azeotropic point at 0.383. The monomer sequence distribution indicated that butadiene could undergo self-propagation to form long flexible segments while dibutyl itaconate preferred to form short sequences with isolated, diad or triad moieties. The mechanical properties of PDIB could compete with or even surpass those of traditional synthetic rubbers. The stress and the elongation at break of unfilled PDIB40 (40 wt% butadiene) were over 2 MPa and 600%, respectively, indicating that PDIB elastomers could be good candidates for the replacement of traditional synthetic rubbers based on fossil resources.


 Please wait while we load your content...
Please wait while we load your content...