Comparing photoswitching of acrylate or methacrylate polymers conjugated with donor–acceptor Stenhouse adducts†
Abstract
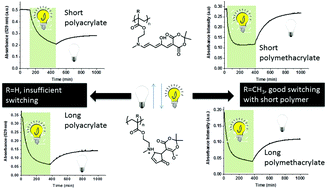
Polymers with donor–acceptor Stenhouse adduct (DASA) groups were synthesized using RAFT methods to evaluate the effect of polymer length (20 vs. 100 DP units) and backbone rigidity (acrylate and methacrylate blocks). A model DASA was not able to switch in water, however once the DASA is conjugated into a polymer, it shows a reversible switching in water. The backbone and chain length of the polymer have a significant effect on the switching ability of the DASA, where only the short methacrylate polymer (pMAEMA29 DASA, sample 3A) shows a fast photoinduced isomerization, rapid thermal recovery and good fatigue resistance. A block copolymer was synthesized with methacrylate polymers containing DASA units by chain extension with a pPEGMEMA block (pPEGMEMA20-b-pMAEMA29, sample 4). The hydrophilic pPEGMEMA block stabilizes the colored isomer of the DASA in DMSO solution relative to both the homopolymer and the free model DASA. Relatively small changes in local environment caused by polymer length results in significant changes in the switching properties of the DASA units decorating the polymer chain, highlighting that detailed understanding of DASA behavior is recommended before applying these in more complex systems.


 Please wait while we load your content...
Please wait while we load your content...