Synthetic mimics of cyclic antimicrobial peptides via templated ring-opening metathesis (TROM)†
Abstract
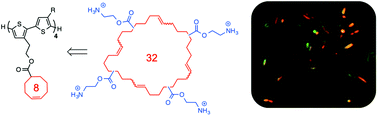
We utilized a templated ring-opening metathesis (TROM) strategy to synthesize a series of precision macrocyclic olefins, each containing two, three or four repeating units of a cyclooctene with pendant carboxylic acid side chains. The structures were confirmed by a combination of NMR spectroscopy, MALDI, and MALDI ms/ms fragmentation studies. In accordance with previous work, we found that cyclooctene monomers covalently tethered to precision oligo(thiophene)s yield exclusively macrocyclic products when subjected to the Grubbs 3rd generation catalyst in highly dilute solution (10−4 M in DCM, 0 °C). Upon hydrolytic liberation of the daughter oligo(olefin) product, further derivatization with cationic groups confers antibacterial and hemolytic activities. We compare the biological activity of these precision macrocycles to that of a polydisperse sample prepared by direct ROM in the absence of a template. Surprisingly, the relatively ill-defined, disperse mixture of oligomeric species exerted biological activity comparable to that of the precision oligomeric macrocycles, suggesting a remarkable degree of tolerance for heterogeneity. These findings provide nuance to the structure–activity relationships understood thus far for AMPs and their mimics, especially in the context of relatively underexplored macrocyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...