Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposite synthesis†
Abstract
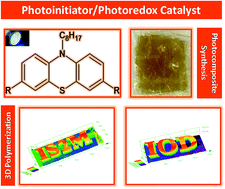
This study highlights the use of four new phenothiazine derivatives (denoted as B compounds: B106, B107, B108, and B111), which are synthesized and proposed as high performance visible light photoinitiators, in combination with an iodonium salt, in both the free radical polymerization (FRP) of acrylates and the cationic polymerization (CP) of epoxides upon visible light exposure using a cheap and safe Light Emitting Diode (LED) at 405 nm. Remarkably, two of them (B108 and B111) are also evaluated as photoredox catalysts (PCs). These phenothiazine compounds can also be used in three-component photoinitiating systems with an iodonium salt and an amine (e.g. N-phenylglycine (NPG)) for the FRP of acrylates upon irradiation with a LED@405 nm. They showed an outstanding polymerization photoinitiation ability; that is, excellent polymerization rates and high final reactive function conversions were obtained. The photoinitiation mechanisms were investigated through different techniques including RT-FTIR, UV–visible spectroscopy, fluorescence spectroscopy, cyclic voltammetry and electron paramagnetic resonance. A full picture of the involved photochemical mechanisms is provided. As high performance photoinitiators, phenothiazine-based systems are especially used in new radical photosensitive 3D printing resins to improve the depth of cure, ensuring the formation of very thick 3D printed polymers. Finally, the use of these new phenothiazine derivatives for the access to thick glass fiber composites using a near-UV conveyor (LED@395 nm) is outlined i.e. thick and filled samples again requiring improved depth of cure.


 Please wait while we load your content...
Please wait while we load your content...