Stereocomplexation of cyclic polylactides with each other and with linear poly(l-lactide)s†
Abstract
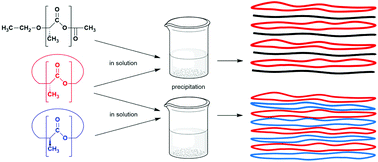
Two kinds of cyclic poly(D- and L-lactide)s were synthesized, namely CI labeled samples mainly consisting of even-numbered cycles with low dispersity and CII, CIII or CIV-labeled ones consisting of equal amounts of even and odd-numbered cycles with high dispersity and higher molecular weights (Mw up to 300 000). Furthermore, linear poly(L-lactide)s were prepared by initiation with ethanol and in both series the molecular weight was varied. The formation of stereocomplexes from cyclic poly(D-lactide)s and all kinds of poly(L-lactide)s was performed in dichloromethane/toluene mixtures. The stereocomplexes crystallized from the reaction mixture were characterized in the virgin state and after annealing at 205 °C. Stereocomplexes free of stereohomopolymers with crystallinities up to 80% were obtained from all experiments in yields ranging from 60 to 80%. Despite the high annealing temperature (maintained for 1 h), little transesterification was observed and the crystallinity slightly increased.


 Please wait while we load your content...
Please wait while we load your content...