A mild and quantitative route towards well-defined strong anionic/hydrophobic diblock copolymers: synthesis and aqueous self-assembly†
Abstract
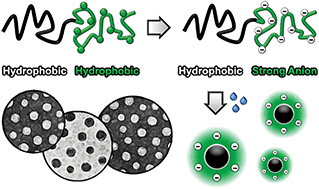
Block copolymers that accommodate both hydrophobic and ionic elements are interesting materials for numerous applications, such as stabilizing agents, lubricants and proton-exchange membranes. Frequently these copolymers are based on weak polyelectrolytes, but the pH-dependent charge density restricts their use to a limited pH window. Although strong polyelectrolytes do not suffer this problem, the most commonly employed post-modification approach limits the choice of the hydrophobic component, as harsh reaction conditions are usually involved. Moreover, this method often results in incomplete functionalization of the precursor copolymer. To avoid these difficulties a mild route was developed that is based on a hydrophobic protected poly(3-sulfopropyl methacrylate) intermediate that enables the preparation of well-defined strong anionic polyelectrolytes. The potential of this method was demonstrated by synthesizing hydrophobic/strong anionic diblock copolymers, and their self-assembly in aqueous solution was studied.


 Please wait while we load your content...
Please wait while we load your content...