Thiol–yne chemistry for 3D printing: exploiting an off-stoichiometric route for selective functionalization of 3D objects†
Abstract
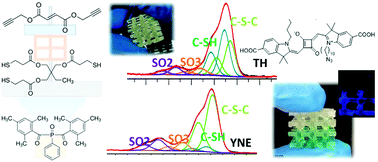
An alkyne monomer bis(propargyl) fumarate is synthesized and used to produce DLP-3D printable formulations with a thiol exploiting photoinduced thiol–yne reaction. By preparing mixtures containing different relative ratios of two monomers, it is possible to obtain polymers exposing different unreacted functional groups on their surface. The reactivity of the formulations toward visible light irradiation is studied by ATR analysis and photorheology tests, the printability with DLP equipment is demonstrated and the thermomechanical properties of the obtained polymers are investigated by DMA. Changing the printing formulation during the printing process, objects exposing either triple bonds or thiol groups or a mix of them can be obtained, and the presence of unreacted groups is confirmed by XPS analysis; this property can be exploited to selectively functionalize the built parts in a dedicated post process. As a proof of concept, a simple hybrid structure is treated with an azide-terminated squaraine dye, to exploit a ‘click’ reaction with the alkyne groups available. The fluorescence of the functionalized structure is observed with a spinning disk confocal microscope. Such a strategy can allow producing 3D objects with a controllable structure just by varying the relative ratio of the co-monomers in the formulations during the printing process.
- This article is part of the themed collection: Chemical Advances in Additive Manufacturing


 Please wait while we load your content...
Please wait while we load your content...